How COVID-19 Vaccine Can Destroy Your Immune System
According to a study that examined how informed consent is given to COVID-19 vaccine trial participants, disclosure forms fail to inform volunteers that the vaccine might make them susceptible to more severe disease if they’re exposed to the virus.
The study,1 “Informed Consent Disclosure to Vaccine Trial Subjects of Risk of COVID-19 Vaccine Worsening Clinical Disease,” published in the International Journal of Clinical Practice, October 28, 2020, points out that “COVID-19 vaccines designed to elicit neutralizing antibodies may sensitize vaccine recipients to more severe disease than if they were not vaccinated.”
“Vaccines for SARS, MERS and RSV have never been approved, and the data generated in the development and testing of these vaccines suggest a serious mechanistic concern: that vaccines designed empirically using the traditional approach (consisting of the unmodified or minimally modified coronavirus viral spike to elicit neutralizing antibodies), be they composed of protein, viral vector, DNA or RNA and irrespective of delivery method, may worsen COVID-19 disease via antibody-dependent enhancement (ADE),” the paper states.
“This risk is sufficiently obscured in clinical trial protocols and consent forms for ongoing COVID-19 vaccine trials that adequate patient comprehension of this risk is unlikely to occur, obviating truly informed consent by subjects in these trials.
The specific and significant COVID-19 risk of ADE should have been and should be prominently and independently disclosed to research subjects currently in vaccine trials, as well as those being recruited for the trials and future patients after vaccine approval, in order to meet the medical ethics standard of patient comprehension for informed consent.”
What Is Antibody-Dependent Enhancement?
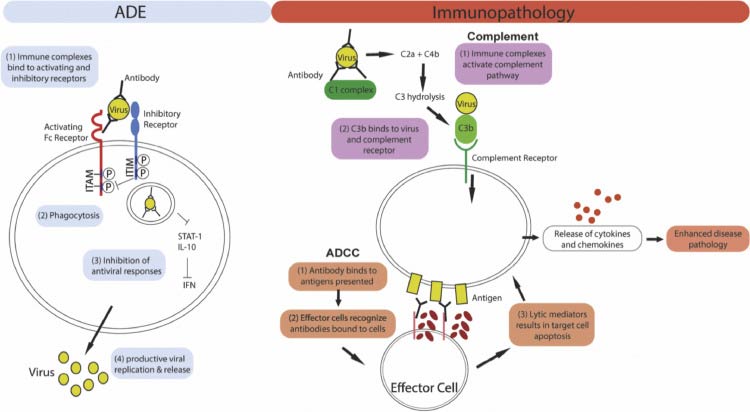
As noted by the authors of that International Journal of Clinical Practice paper, previous coronavirus vaccine efforts — for severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV) and respiratory syncytial virus (RSV) — have revealed a serious concern: The vaccines have a tendency to trigger antibody-dependent enhancement.
What exactly does that mean? In a nutshell, it means that rather than enhance your immunity against the infection, the vaccine actually enhances the virus’ ability to enter and infect your cells, resulting in more severe disease than had you not been vaccinated.2
This is the exact opposite of what a vaccine is supposed to do, and a significant problem that has been pointed out from the very beginning of this push for a COVID-19 vaccine. The 2003 review paper “Antibody-Dependent Enhancement of Virus Infection and Disease” explains it this way:3
“In general, virus-specific antibodies are considered antiviral and play an important role in the control of virus infections in a number of ways. However, in some instances, the presence of specific antibodies can be beneficial to the virus. This activity is known as antibody-dependent enhancement (ADE) of virus infection.
The ADE of virus infection is a phenomenon in which virus-specific antibodies enhance the entry of virus, and in some cases the replication of virus, into monocytes/macrophages and granulocytic cells through interaction with Fc and/or complement receptors.
This phenomenon has been reported in vitro and in vivo for viruses representing numerous families and genera of public health and veterinary importance. These viruses share some common features such as preferential replication in macrophages, ability to establish persistence, and antigenic diversity. For some viruses, ADE of infection has become a great concern to disease control by vaccination.”
Previous Coronavirus Vaccine Efforts Have All Failed
In my May 2020 interview above with Robert Kennedy Jr., he summarized the history of coronavirus vaccine development, which began in 2002, following three consecutive SARS outbreaks. By 2012, Chinese, American and European scientists were working on SARS vaccine development, and had about 30 promising candidates.
Of those, the four best vaccine candidates were then given to ferrets, which are the closest analogue to human lung infections. In the video below, which is a select outtake from my full interview, Kennedy explains what happened next. While the ferrets displayed robust antibody response, which is the metric used for vaccine licensing, once they were challenged with the wild virus, they all became severely ill and died.
The same thing happened when they tried to develop an RSV vaccine in the 1960s. RSV is an upper respiratory illness that is very similar to that caused by coronaviruses. At that time, they had decided to skip animal trials and go directly to human trials.
“They tested it on I think about 35 children, and the same thing happened,” Kennedy said. “The children developed a champion antibody response — robust, durable. It looked perfect [but when] the children were exposed to the wild virus, they all became sick. Two of them died. They abandoned the vaccine. It was a big embarrassment to FDA and NIH.”
Neutralizing Versus Binding Antibodies
Coronaviruses produce not just one but two different types of antibodies:
- Neutralizing antibodies,4 also referred to as immoglobulin G (IgG) antibodies, that fight the infection
- Binding antibodies5 (also known as nonneutralizing antibodies) that cannot prevent viral infection
Instead of preventing viral infection, binding antibodies trigger an abnormal immune response known as “paradoxical immune enhancement.” Another way to look at this is your immune system is actually backfiring and not functioning to protect you but actually making you worse.
Many of the COVID-19 vaccines currently in the running are using mRNA to instruct your cells to make the SARS-CoV-2 spike protein (S protein). The spike protein, which is what attaches to the ACE2 receptor of the cell, is the first stage of the two-stage process viruses use to gain entry into cells.
The idea is that by creating the SARS-CoV-2 spike protein, your immune system will commence production of antibodies, without making you sick in the process. The key question is, which of the two types of antibodies are being produced through this process?
Without Neutralizing Antibodies, Expect More Severe Illness
In an April 2020 Twitter thread,6 The Immunologist noted: “While developing vaccines … and considering immunity passports, we must first understand the complex role of antibodies in SARS, MERS and COVID-19.” He goes on to list several coronavirus vaccine studies that have raised concerns about ADE.
The first is a 2017 study7 in PLOS Pathogens, ”Enhanced Inflammation in New Zealand White Rabbits When MERS-CoV Reinfection Occurs in the Absence of Neutralizing Antibody,” which investigated whether getting infected with MERS would protect the subject against reinfection, as is typically the case with many viral illnesses. (Meaning, once you recover from a viral infection, say measles, you’re immune and won’t contract the illness again.)
To determine how MERS affects the immune system, the researchers infected white rabbits with the virus. The rabbits got sick and developed antibodies, but those antibodies were not the neutralizing kind, meaning the kind of antibodies that block infection. As a result, they were not protected from reinfection, and when exposed to MERS for a second time, they became ill again, and more severely so.
“In fact, reinfection resulted in enhanced pulmonary inflammation, without an associated increase in viral RNA titers,” the authors noted. Interestingly, neutralizing antibodies were elicited during this second infection, preventing the animals from being infected a third time. According to the authors:
“Our data from the rabbit model suggests that people exposed to MERS-CoV who fail to develop a neutralizing antibody response, or persons whose neutralizing antibody titers have waned, may be at risk for severe lung disease on re-exposure to MERS-CoV.”
In other words, if the vaccine does not result in a robust response in neutralizing antibodies, you might be at risk for more severe lung disease if you’re infected with the virus.
And here’s an important point: COVID-19 vaccines are NOT designed to prevent infection. As detailed in “How COVID-19 Vaccine Trials Are Rigged,” a “successful” vaccine merely needs to reduce the severity of the symptoms. They’re not even looking at reducing infection, hospitalization or death rates.
ADE in Dengue Infections
The Dengue virus is also known to cause ADE. As explained in a Swiss Medical Weekly paper published in April 2020:8
“The pathogenesis of COVID-19 is currently believed to proceed via both directly cytotoxic and immune-mediated mechanisms. An additional mechanism facilitating viral cell entry and subsequent damage may involve the so-called antibody-dependent enhancement (ADE).
ADE is a very well-known cascade of events whereby viruses may infect susceptible cells via interaction between virions complexed with antibodies or complement components and, respectively, Fc or complement receptors, leading to the amplification of their replication.
This phenomenon is of enormous relevance not only for the understanding of viral pathogenesis, but also for developing antiviral strategies, notably vaccines …
There are four serotypes of Dengue virus, all eliciting protective immunity. However, although homotypic protection is long-lasting, cross-neutralizing antibodies against different serotypes are short-lived and may last only up to 2 years.
In Dengue fever, reinfection with a different serotype runs a more severe course when the protective antibody titer wanes. Here, non-neutralizing antibodies take over neutralizing ones, bind to Dengue virions, and these complexes mediate the infection of phagocytic cells via interaction with the Fc receptor, in a typical ADE.
In other words, heterotypic antibodies at subneutralizing titres account for ADE in persons infected with a serotype of Dengue virus that is different from the first infection.
Cross-reactive neutralizing antibodies are associated with decreased odds of symptomatic secondary infection, and the higher the titer of such antibodies following the primary infection, the longer the delay to symptomatic secondary infection …”
The paper goes on to detail results from follow-up investigations into the Dengue vaccine, which revealed the hospitalization rate for Dengue among vaccinated children under the age of 9 was greater than the rate among controls. The explanation for this appears to be that the vaccine mimicked a primary infection, and as that immunity waned, the children became susceptible to ADE when they encountered the virus a second time. The author explains:
“A post hoc analysis of efficacy trials, using an anti-nonstructural protein 1 immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) to distinguish antibodies elicited by wild-type infection from those following vaccination, showed that the vaccine was able to protect against severe Dengue [in] those who had been exposed to the natural infection before vaccination, and that the risk of severe clinical outcome was increased among seronegative persons.
Based on this, a Strategic Advisor Group of Experts convened by World Health Organization (WHO) concluded that only Dengue seropositive persons should be vaccinated whenever Dengue control programs are planned that include vaccination.”
ADE in Coronavirus Infections
This could end up being important for the COVID-19 vaccine. Hypothetically speaking, if SARS-CoV-2 works like Dengue, which is also caused by an RNA virus, then anyone who has not tested positive for SARS-CoV-2 might actually be at increased risk for severe COVID-19 after vaccination, and only those who have already recovered from a bout of COVID-19 would be protected against severe illness by the vaccine.
To be clear, we do not know whether that is the case or not, but these are important areas of inquiry and the current vaccine trials will simply not be able to answer this important question.
The Swiss Medical Weekly paper9 also reviews the evidence of ADE in coronavirus infections, citing research showing inoculating cats against the feline infectious peritonitis virus (FIPV) — a feline coronavirus — increases the severity of the disease when challenged with the same FIPV serotype as that in the vaccine.
The paper also cites research showing “Antibodies elicited by a SARS-CoV vaccine enhanced infection of B cell lines in spite of protective responses in the hamster model.” Another paper,10 “Antibody-Dependent SARS Coronavirus Infection Is Mediated by Antibodies Against Spike Proteins,” published in 2014, found that:
“… higher concentrations of anti-sera against SARS-CoV neutralized SARS-CoV infection, while highly diluted anti-sera significantly increased SARS-CoV infection and induced higher levels of apoptosis.
Results from infectivity assays indicate that SARS-CoV ADE is primarily mediated by diluted antibodies against envelope spike proteins rather than nucleocapsid proteins. We also generated monoclonal antibodies against SARS-CoV spike proteins and observed that most of them promoted SARS-CoV infection.
Combined, our results suggest that antibodies against SARS-CoV spike proteins may trigger ADE effects. The data raise new questions regarding a potential SARS-CoV vaccine …”
A study11 that ties into this was published in the journal JCI Insight in 2019. Here, macaques vaccinated with a modified vaccinia Ankara (MVA) virus encoding full-length SARS-CoV spike protein ended up with more severe lung pathology when the animals were exposed to the SARS virus. And, when they transferred anti-spike IgG antibodies into unvaccinated macaques, they developed acute diffuse alveolar damage, likely by “skewing the inflammation-resolving response.”
SARS Vaccine Worsens Infection After Challenge With SARS-CoV
An interesting 2012 paper12 with the telling title, “Immunization with SARS Coronavirus Vaccines Leads to Pulmonary Immunopathology on Challenge with the SARS Virus,” demonstrates what many researchers now fear, namely that COVID-19 vaccines may end up making people more prone to severe SARS-CoV-2 infection.
The paper reviews experiments showing immunization with a variety of SARS vaccines resulted in pulmonary immunophathology once challenged with the SARS virus. As noted by the authors:13
“Inactivated whole virus vaccines whether inactivated with formalin or beta propiolactone and whether given with our without alum adjuvant exhibited a Th2-type immunopathologic in lungs after challenge.
As indicated, two reports attributed the immunopathology to presence of the N protein in the vaccine; however, we found the same immunopathologic reaction in animals given S protein vaccine only, although it appeared to be of lesser intensity.
Thus, a Th2-type immunopathologic reaction on challenge of vaccinated animals has occurred in three of four animal models (not in hamsters) including two different inbred mouse strains with four different types of SARS-CoV vaccines with and without alum adjuvant. An inactivated vaccine preparation that does not induce this result in mice, ferrets and nonhuman primates has not been reported.
This combined experience provides concern for trials with SARS-CoV vaccines in humans. Clinical trials with SARS coronavirus vaccines have been conducted and reported to induce antibody responses and to be ‘safe.’ However, the evidence for safety is for a short period of observation.
The concern arising from the present report is for an immunopathologic reaction occurring among vaccinated individuals on exposure to infectious SARS-CoV, the basis for developing a vaccine for SARS. Additional safety concerns relate to effectiveness and safety against antigenic variants of SARS-CoV and for safety of vaccinated persons exposed to other coronaviruses, particularly those of the type 2 group.”
The Elderly Are Most Vulnerable to ADE
On top of all of these concerns, there’s evidence showing the elderly — who are most vulnerable to severe COVID-19 — are also the most vulnerable to ADE. Preliminary research findings14 posted on the preprint server medRxiv at the end of March 2020 reported that middle-aged and elderly COVID-19 patients have far higher levels of anti-spike antibodies — which, again, increase infectivity — than younger patients.
Immune Enhancement Is a Serious Concern
Another paper worth mentioning is the May 2020 mini review15 “Impact of Immune Enhancement on COVID-19 Polyclonal Hyperimmune Globulin Therapy and Vaccine Development.” As in many other papers, the authors point out that:16
“While development of both hyperimmune globulin therapy and vaccine against SARS-CoV-2 are promising, they both pose a common theoretical safety concern. Experimental studies have suggested the possibility of immune-enhanced disease of SARS-CoV and MERS-CoV infections, which may thus similarly occur with SARS-CoV-2 infection …
Immune enhancement of disease can theoretically occur in two ways. Firstly, non-neutralizing or sub-neutralizing levels of antibodies can enhance SARS-CoV-2 infection into target cells.
Secondly, antibodies could enhance inflammation and hence severity of pulmonary disease. An overview of these antibody dependent infection and immunopathology enhancement effects are summarized in Fig. 1 …
Currently, there are multiple SARS-CoV and MERS-CoV vaccine candidates in pre-clinical or early phase clinical trials. Animal studies on these CoVs have shown that the spike (S) protein-based vaccines (specifically the receptor binding domain, RBD) are highly immunogenic and protective against wild-type CoV challenge.
Vaccines that target other parts of the virus, such as the nucleocapsid, without the S protein, have shown no protection against CoV infection and increased lung pathology. However, immunization with some S protein based CoV vaccines have also displayed signs of enhanced lung pathology following challenge.
Hence, besides the choice of antigen target, vaccine efficacy and risk of immunopathology may be dependent on other ancillary factors, including adjuvant formulation, age at vaccination … and route of immunization.”
Do a Risk-Benefit Analysis Before Making Up Your Mind
In all likelihood, regardless of how effective (or ineffective) the COVID-19 vaccines end up being, they’ll be released to the public in relatively short order. Most predict one or more vaccines will be ready sometime in 2021.
Ironically, the data17,18,19 we now have no longer support a mass vaccination mandate, considering the lethality of COVID-19 is lower than the flu for those under the age of 60.20 If you’re under the age of 40, your risk of dying from COVID-19 is just 0.01%, meaning you have a 99.99% chance of surviving the infection. And you could improve that to 99.999% if you’re metabolically flexible and vitamin D replete.
So, really, what are we protecting against with a COVID-19 vaccine? As mentioned, the vaccines aren’t even designed to prevent infection, only reduce the severity of symptoms. Meanwhile, they could potentially make you sicker once you’re exposed to the virus. That seems like a lot of risk for a truly questionable benefit.
To circle back to where we started, participants in current COVID-19 vaccine trials are not being told of this risk — that by getting the vaccine they may end up with more severe COVID-19 once they’re infected with the virus.
Lethal Th2 Immunopathology Is Another Potential Risk
In closing, consider what this PNAS news feature states about the risk of vaccine-induced immune enhancement and dysfunction, particularly for the elderly, the very people who would need the protection a vaccine might offer the most:21
“Since the 1960s, tests of vaccine candidates for diseases such as dengue, respiratory syncytial virus (RSV), and severe acute respiratory syndrome (SARS) have shown a paradoxical phenomenon:
Some animals or people who received the vaccine and were later exposed to the virus developed more severe disease than those who had not been vaccinated. The vaccine-primed immune system, in certain cases, seemed to launch a shoddy response to the natural infection …
This immune backfiring, or so-called immune enhancement, may manifest in different ways such as antibody-dependent enhancement (ADE), a process in which a virus leverages antibodies to aid infection; or cell-based enhancement, a category that includes allergic inflammation caused by Th2 immunopathology. In some cases, the enhancement processes might overlap …
Some researchers argue that although ADE has received the most attention to date, it is less likely than the other immune enhancement pathways to cause a dysregulated response to COVID-19, given what is known about the epidemiology of the virus and its behavior in the human body.
‘There is the potential for ADE, but the bigger problem is probably Th2 immunopathology,’ says Ralph Baric, an epidemiologist and expert in coronaviruses … at the University of North Carolina at Chapel Hill.
In previous studies of SARS, aged mice were found to have particularly high risks of life-threatening Th2 immunopathology ... in which a faulty T cell response triggers allergic inflammation, and poorly functional antibodies that form immune complexes, activating the complement system and potentially damaging the airways.”
from Articles https://ift.tt/36nNqD4
via IFTTT